Safer Meds
Secure medicine take-back programs. Pharmaceutical stewardship policies. Reducing medicine abuse & poisonings. Reducing pharmaceutical pollution.How Community Environmental Health Strategies works for Safer Meds
- Providing policy expertise and process guidance to develop pharmaceutical stewardship policies that provide sustainable financing for comprehensive and convenient secure medicine return systems.
- Clarifying the regulatory context for safe disposal of unwanted medicines.
- Supporting expansion of secure drug take-back programs for leftover prescription and over-the-counter medicines to protect our families and our environment.
Prescription and over-the-counter medicines can improve our health when they’re used properly. But abuse, poisonings and overdoses from these medicines is a serious problem. The home medicine cabinet has become the new drug dealer, contributing to our nation’s prescription drug abuse crisis. It’s not just an opioid problem – other prescription and over-the-counter medicines are also misused.
About one-third of medicines sold go unused for many reasons. Unwanted medicines in our homes increase risks of medicine misuse and poisonings. Flushing waste medicines down the drain or putting medicines in the household trash adds to pharmaceutical pollution in our waterways. Secure medicine take-back programs are the solution to these problems.
Secure medicine take-back programs are part of a comprehensive approach to preventing medicine abuse and unintentional poisonings. And proper disposal keeps waste medicines from contributing to pharmaceutical pollution.
In pharmaceutical stewardship policies, the companies that produce the medicines finance and coordinate a convenient system for secure collection and environmentally sound disposal of leftover medicines. Robust and comprehensive medicine take-back program can be provided through this dedicated funding from pharmaceutical manufacturers. Protecting our families and our environment.
Pharmaceutical Stewardship Policies
Medicine Take-back Programs
Regulatory Considerations
It’s a Go! WA’s Statewide Pharmaceutical Stewardship Program Launched!
Washington State Celebrates the Official Start of the First Statewide, Comprehensive Drug Take-Back Program Operated by the Pharmaceutical Industry! A fitting subtitle is "good things come to those who organize and persist". It took many years of educating,...
Pharmaceutical Stewardship Passes in Oregon, 4th Law in Nation!
A fourth state Legislature has voted overwhelmingly for a pharmaceutical stewardship, a.k.a. extended producer responsibility, approach to providing convenient drug take-back services to all residents. After many years of discussion on the issue, the Oregon...
Another look at medicine disposal products: performance and effectiveness?
My latest dive into assessing whether medicine disposal products provide a safe, effective, and environmentally sound way to dispose of pharmaceuticals is now out in report form. Medicine Disposal Products: an overview of products & performance questions examines...
National Association of Counties article on WA drug take-back successes!
Thanks to NACo for helping share the news about successful pharmaceutical stewardship laws in Washington State. NACo County News published my article explaining how local policies served as innovative models and drove the adoption of WA's statewide law. ...
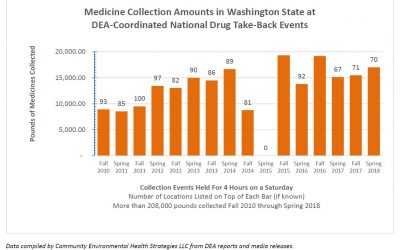
15th National DEA Drug Take-Back Event Captures Almost 1 Million Pounds!
In just 4 hours on Saturday April 28th, people across the U.S. returned almost 1 million pounds of leftover and expired medicines for safe disposal. The 15th National DEA Drug Take-Back Day netted 949,046 pounds of drugs or 475.5 tons! Complete results of the...
Secure Drug Take-Back Act Becomes Law in WA!
Proponents of pharmaceutical stewardship in Washington State are celebrating a new law to help reduce medicine misuse, poisonings, overdoses, and that will also help reduce pharmaceutical pollution - the WA Secure Drug Take-Back Act. This...
What About Medicine Disposal Products? Effective? Safe? Worth the Price?
New products in pouches and bottles are being marketed to consumers with claims that they provide a easy way to "denature" drugs and make them safe to toss in the household trash. Some companies have formed partnerships with elected officials to distribute free...
Local Pharmaceutical Stewardship Laws Achieve Their Promise
When counties in California and Washington state, like Alameda and King, began enacting pharmaceutical stewardship policies several years ago it was out of the compelling need to provide more medicine take-back services to their residents. Now in 2017, the robust and...
WA Secure Drug Take-Back Act Advances Against Pharmaceutical Industry Opposition
The Washington State Legislature has an opportunity to act on a public safety bill that helps address the epidemic of opioid and medicine abuse and overdose deaths in our communities by creating a statewide medicine take-back program. The WA Secure Drug Take-back...
Kitsap and Pierce Counties Take Action on Secure Medicine Return
Two more local Boards of Health in WA state took action last week to provide their communities with more secure and environmentally sound disposal systems for leftover prescription and over-the-counter medications. On Dec. 6th, the Kitsap County Public Health board...
Without adequate and sustainable financing, secure medicine return programs cannot expand to more convenient locations, do not have resources for effective education and promotion, and don’t have the capacity to handle the large amounts of leftover medicines from households.
Margaret Shield’s experience with Pharmaceutical Stewardship.
- Policy and advocacy leader of the successful campaign to pass Washington State’s Secure Drug Take-Back Act in 2018!
- Ten years of legislative and regulatory work at the state, local and federal levels, including researching the problem, crafting policy solutions, writing legislative language, building a diverse coalition of advocates, engaging stakeholders, and leading legislative campaigns.
- Lead policy staff for development of the King County Board of Health’s Secure Medicine Return Regulations, the model for other county ordinances in Washington and California.
- Policy consultant to four local health jurisdictions for development of their Secure Medicine Return Regulations: the Snohomish Health District, the Tacoma-Pierce County Health Department, the Whatcom County Health Department and the Skagit County Public Health Department.
Learn more about WA’s local drug take-back laws > - Currently working with local organizations and advocates across the country on policy development and implementation.
- Helped lead national efforts to remove unintended barriers to take-back of controlled substances that resulted in passage of the Secure & Responsible Drug Disposal Act of 2010 and the DEA’s Rule in 2014.
- Collaborating with experts and advocates across Washington State and the U.S. since 2008 on pharmaceutical stewardship policies, medicine take-back programs, and preventing medicine misuse and pharmaceutical pollution.
In 2018, the successful local policy model in Washington State was translated to the state level with passage of the WA Secure Drug Take-Back Act.
Resources
These older handouts provide a snapshot of secure medicine return services being provided under county-level pharmaceutical stewardship ordinances in Washington and California.
Secure Medicine Return Laws in WA State (PDF, 2 pages)
summarizes implementation status of county-level pharmaceutical stewardship laws; lists number of secure drop boxes as of January 2019.
Implementation Status of County Pharmaceutical Stewardship Ordinances (CA, WA, NY) Oct. 2017
Pharmaceutical Stewardship Policy Update – October 2016
This policy brief provides an overview of county-level pharmaceutical stewardship policies and a history of implementation of the first ordinances in Alameda and King counties. Last updated in October 2016, it does not describe the additional local regulations enacted during late 2016 and 2017.
Resources provided on this site may not be used for commercial purposes, reused without attribution, or modified for reuse. Please link to this site for the latest updates to these Resources.
Want to understand pharmaceutical stewardship policies? Or working on a policy for your community?
Contact me to discuss how I can help make your policy process successful by:
- explaining the pharmaceutical stewardship policy approach to comprehensive and sustainably financed programs.
- providing comprehensive background and analysis on key decision points and common questions.
- examining policy options and customizing legislative language for your community.
- addressing medicine take-back operations and key regulatory issues in the context of policy development.
- developing an effective policy communications and outreach strategy.
Learn more about my Services
Estimates are that one-third of prescription and over-the-counter medicines sold go unused. Unwanted medicines in our homes increase risks of medicine misuse and poisonings. Improper disposal of medicines down the drain or in the household trash adds to pharmaceutical pollution in the environment.
Take-back programs collect unused medicines securely and destroy them safely at properly permitted incineration facilities. The FDA, DEA, and the EPA all recommend the use of medicine take-back programs as the safe way to dispose of leftover medicines. Medicine take-back is safer than throwing medicines in the household trash, which is not secure, not recommended for controlled substances by the DEA, and does not ensure the pharmaceuticals won’t be released into the environment through landfill leachate.
Medicine take-back programs work, but often are not available in all communities or cannot support enough convenient drop-off locations. Existing programs struggle for ongoing financing and are a burden on over-stretched local law enforcement, city, and county budgets. Some jurisdictions are adopting pharmaceutical stewardship policies to require medicine manufacturers to finance and coordinate drug take-back programs, as they already do in other countries.
Exploring medicine take-back program options for your community?
- public health and environmental concerns with leftover medicines.
- options for secure collection, transport, and environmentally sound disposal.
- key regulatory considerations.
- costs and funding considerations.
- policy and legislative approaches for comprehensive and sustainable programs.
Learn more about my Services
In recent years, there have been key developments in this arena to remove unintentional regulatory barriers, notably the DEA’s Rule on Disposal of Controlled Substances as well as changes to many state laws. These changes make it easier to operate convenient and secure medicine return programs.
Prior to the DEA Rule for Disposal of Controlled Substances, leftover or expired controlled substances – such as prescription narcotics and stimulants – could only be legally collected from residents by law enforcement. Through collection events or drop-off programs.
The Secure and Responsible Drug Disposal Act was signed into law in October 2010. It amended the Controlled Substances Act and authorized the DEA to develop the new Rule, which was finally issued in October 2014. The law and the Rule remove key barriers to operating take-back programs, but do not mandate the creation of any medicine take-back programs, nor does it provide any funding for those programs. The Rule expands allowed options for collecting controlled substances from individuals and their family members for safe destruction by secure medicine return programs. The new options are more convenient for residents and even more effective in reducing misuse or improper disposal of leftover household medicines.
Trying to make sense of the regulations?
Learn more about my Services